Gate2Compliance™ (formerly Gate2GMP®) is an essential subscription service for Life science professionals working with GMP, Quality Systems and regulatory compliance. The service is sponsored by Key2Compliance®.
The main feature is a single source library with easy access to regulatory requirements, FDA warning letters and interpretation documents. Gate2Compliance™ contains the most comprehensive collection of said documents and is constantly updated and maintained to provide users with correct information.
Premium
With a Premium account, you will get extended search functions, a unique database with FDA Warning Letters, searchable both on all paragraphs in 21 CFR 211 and 21 CFR 820 respectively, but also free search in Warning Letters.
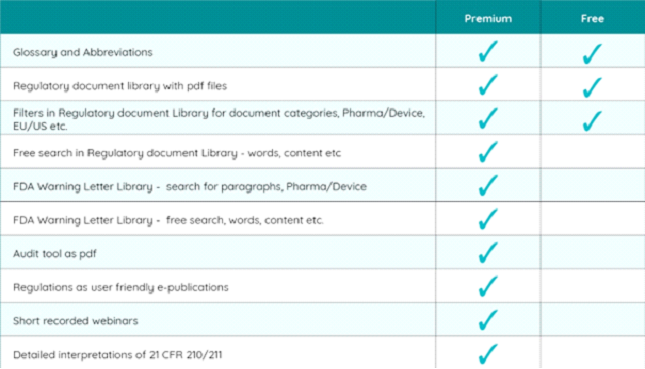
Another unique feature is detailed pharma CGMP interpretation tool (21 CFR 210/211) providing FDAs own interpretations of their regulatory paragraphs.
You also get e-publications (audit tool as pdf, GMPs and regulations as easy-to-use electronic files) and recorded short webinars.
All of this is available for only 295 €/year. Read more and subscribe here >

Key2Compliance® focuses on assisting companies in the Life Science area. We offer complete solutions in all aspects of our customers’ needs. Our services include both training and consultancy related to Pharmaceuticals and Medical Device products throughout the full product life cycle.



